Edge Article
The Reductive Disproportionation of CO2 using a Magnesium(I) Complex: Analogies with Low Valent f-Block Chemistry
Chem. Sci., 2013, Accepted Manuscript
DOI: 10.1039/C3SC52242CReceived 10 Aug 2013, Accepted 30 Aug 2013
First published online 30 Aug 2013
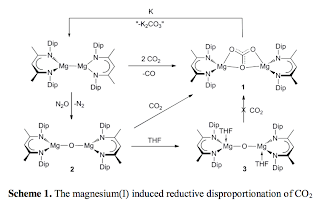
The dimeric magnesium(I) complex, [{(DipNacnac)Mg}2] (DipNacnac = [(DipNCMe)2CH]-, Dip = C6H3Pri2-2,6), reacts with 2 equivalents of CO2 to give a high yield of the MgII carbonate complex, [{(DipNacnac)Mg}2(-2:2-CO3)], and CO via a reductive disproportionation process. The MgII oxalate, [{(DipNacnac)Mg}2(-C2O4)], is a very low yield by-product of the reaction. Reducing the carbonate complex with elemental potassium regenerates the MgI starting material. The carbonate complex is shown to form via a stepwise process involving the oxo-bridged intermediate, [{(DipNacnac)Mg}2(-O)], which rapidly reacts with stoichiometric CO2 to give [{(DipNacnac)Mg}2(-2:2-CO3)]. The oxo-bridged intermediate has been rationally synthesised via the reaction of [{(DipNacnac)Mg}2] with N2O, and treated with THF to give the adduct, [{(THF)(DipNacnac)Mg}2(-O)]. The complex, [{(DipNacnac)Mg}2(-O)], reacts with the CO2 isoelectronic analogues, CS2 and CyNCNCy (Cy = cyclohexyl) to give the complexes, [{(DipNacnac)Mg}2(-2:2-CS2O)] and [{(DipNacnac)Mg}2{-2:2-C(NCy)2O}]. Rearrangement of the dithiocarbonate coordination mode of the former occurs upon treatment with diethyl ether, giving the unsymmetrical complex, [{(DipNacnac)Mg}(-2(S,S'):1(O)-CS2O){Mg(DipNacnac)(OEt2)}]. The majority of the complexes prepared in this study were crystallographically characterised. Taken as a whole, this study demonstrates that magnesium(I) dimers can display very similar reactivity, with respect to small molecules activations, as do SmII and UIII compounds. Accordingly, magnesium(I) compounds hold considerable potential as cheaper, less toxic, non-radioactive and diamagnetic alternatives to low valent f-block metal complexes in this realm.
コメント