Capture of CO2 by a Cationic Nickel(I) Complex in the Gas Phase and Characterization of the Bound, Activated CO2 Molecule by Cryogenic Ion Vibrational Predissociation Spectroscopy
, , , , ,, and *
Dr. F. S. Menges, S. M. Craig, N. Tçtsch, Dr. A. Bloomfield,
Prof. Dr. M. A. Johnson
Department of Chemistry, Yale University
225 Prospect St., New Haven, CT 06511 (USA)
S. Ghosh, Prof. Dr. H.-J. Krger
Fachbereich Chemie, Technische Universitt Kaiserslautern
Erwin-Schrçdinger-Strasse, 67663 Kaiserslautern (Germany)
Angew. Chem. Int. Ed. 2015, 54, 1 – 5
http://onlinelibrary.wiley.com/doi/10.1002/anie.201507965/abstract
Abstract
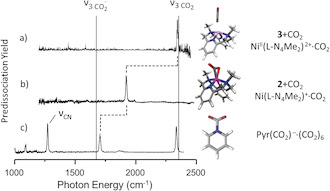
We describe a systematic method for the preparation and spectroscopic characterization of a CO2
molecule coordinated to an activated bisphenoidal nickel(I) compound
containing a tetraazamacrocyclic ligand in the gas phase. The resulting
complex was then structurally characterized by using mass-selected
vibrational predissociation spectroscopy. The results indicate that a
highly distorted CO2 molecule is bound to the metal center in an η2-C,O coordination mode, thus establishing an efficient and rational method for the preparation of metal-activated CO2 for further studies using ion chemistry techniques.
Highly distorted: A CO2 molecule is activated
in the gas phase by a cationic mononuclear nickel(I) complex containing a
tetraazamacrocyclic ligand. The resulting complex was structurally
characterized by mass-selected vibrational predissociation spectroscopy,
which indicated that this complex features a highly distorted CO2 molecule bound to the metal center in an η2-C,O coordination mode.


コメント