Angewandte ChemieBiocatalysis
Peter Both, Hanna Busch, Paul P. Kelly, Francesco G. Mutti, Nicholas J. Turner, and Sabine L. Flitsch*
Peter Both, Hanna Busch, Paul P. Kelly, Francesco G. Mutti, Nicholas J. Turner, and Sabine L. Flitsch*
Abstract
Enantiomerically
pure chiral amines are ubiquitous chemical building blocks in bioactive
pharmaceutical products and their synthesis from simple starting
materials is of great interest. One of the most attractive strategies is
the stereoselective installation of a chiral amine through C![[BOND]](https://lh3.googleusercontent.com/blogger_img_proxy/AEn0k_sI1sKN3yZb78Zb3BdSwhT2dKTaYQ4WTB7k1wdU62O3I8-XUYsai3uRzJTt3a6E1R3RbPiAFJiWQz8PpuMGjHVbDGoZi8uZpeq3Blww87Q-LqdZIyCafaIDG2qoikvnzIAFFQtFLXwx-mdPtA=s0-d) H
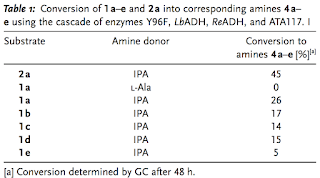
amination, which is a challenging chemical transformation. Herein we
report the application of a multienzyme cascade, generated in a single
bacterial whole-cell system, which is able to catalyze stereoselective
benzylic aminations with ee values of 97.5 %. The cascade uses
four heterologously expressed recombinant enzymes with cofactors
provided by the host cell and isopropyl amine added as the amine donor.
The cascade presents the first example of the successful de novo design
of a single whole-cell biocatalyst for formal stereoselective C
H
amination, which is a challenging chemical transformation. Herein we
report the application of a multienzyme cascade, generated in a single
bacterial whole-cell system, which is able to catalyze stereoselective
benzylic aminations with ee values of 97.5 %. The cascade uses
four heterologously expressed recombinant enzymes with cofactors
provided by the host cell and isopropyl amine added as the amine donor.
The cascade presents the first example of the successful de novo design
of a single whole-cell biocatalyst for formal stereoselective C![[BOND]](https://lh3.googleusercontent.com/blogger_img_proxy/AEn0k_sI1sKN3yZb78Zb3BdSwhT2dKTaYQ4WTB7k1wdU62O3I8-XUYsai3uRzJTt3a6E1R3RbPiAFJiWQz8PpuMGjHVbDGoZi8uZpeq3Blww87Q-LqdZIyCafaIDG2qoikvnzIAFFQtFLXwx-mdPtA=s0-d) H amination.
H amination.




コメント